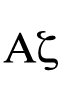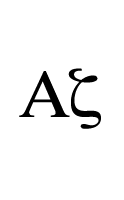[To add to Patchaon's answer.]
On Tue, Dec 11, 2001 at 07:33:22PM -0600, bc wrote:
> question: may have asked this before, but am still very confused
> on the basic relationship between electromagnetic energy and light.
> how are they different and-or similar? i think R. or ~g may have
> already addressed this question before, yet i am still confused.
>
> 'light' is part of the electromagnetic spectrum (EM)
>
> the electromagnetic spectrum, all frequencies, are energy
>
> light is, then, electromagnetic energy (yes/no?)
>
Yes. The more usual way of saying this is that light is
electromagnetic radiation.
> does 'light' exist on the whole EM spectrum, as 'light?',
> as different kinds of light (UV, X-ray...), or is light to be
> confined to a certain spectral analysis that humans can see?
Light is electromagnetic radiation which humans can see--energy in the
"visible spectrum." This varies a bit from person to person.
>
> (for example, when seeing spectral analysis of distant stars,
> there is the rainbow which shows the colors representing a
> series of different elements, such as gases, which are shown
> in red, green, blue vertical bands of light, etc)...
>
Those are visible; if you use a prism or diffraction grating they can
actually be seen. The colors reflect the physical processes which
generate the light. Thus, if you add a small amount of copper to a
gas flame, the flame will turn green.
Sometimes color is also used to represent invisible relationships, of
course.
> if there is an electron in the atom, composing matter, the
> material world, and this has mass and energy, is electromagnetic,
> and some of its effects include giving off 'light' particles, photons,
> when a molecule or an atom's particles are knocked out of orbit,
> how does the photon, particle of light, relate to the electron,
> and these to the electromagnetic spectrum?
Physicists say that photons and electrons are of two different orders
of particles: bosons and fermions. Physicists say that the
distinguishing property of fermions and bosons is "spin"--fermions
have spins of 1/2, 3/2, and so on and bosons have spins of 0, 1, 2,
and so on--but I'm not sure what physicists mean when they talk about
"spin" at the subatomic level; it is not like the particles are
turning objects. At the same time, aggregate enough subatomic spin,
and one will observe macroscopic angular momentum--turning due to
magnetism. I'm not a physicist--I don't understand this. :-)
Anyhow, some of the simpler differences between electrons and photons are:
electron photon
has a rest mass no rest mass
has a charge no charge
participates in stable atoms carries electromagnetic energy
A photon can bump an electron up in orbital energy (by a
fixed--quantized--amount) and be absorbed. The new orbit isn't
stable, and eventually the electron falls back into its old orbit,
emitting another photon. There is no energy loss--there is no
friction at the sub-atomic level.
Electrons can also gain energy in other ways--for instance by
application of an external electric or magnetic field, or from
collisions with other sorts of particles. This is how electric lights
work.
Hope this is helpful,
Randolph
On Tue, Dec 11, 2001 at 07:33:22PM -0600, bc wrote:
> question: may have asked this before, but am still very confused
> on the basic relationship between electromagnetic energy and light.
> how are they different and-or similar? i think R. or ~g may have
> already addressed this question before, yet i am still confused.
>
> 'light' is part of the electromagnetic spectrum (EM)
>
> the electromagnetic spectrum, all frequencies, are energy
>
> light is, then, electromagnetic energy (yes/no?)
>
Yes. The more usual way of saying this is that light is
electromagnetic radiation.
> does 'light' exist on the whole EM spectrum, as 'light?',
> as different kinds of light (UV, X-ray...), or is light to be
> confined to a certain spectral analysis that humans can see?
Light is electromagnetic radiation which humans can see--energy in the
"visible spectrum." This varies a bit from person to person.
>
> (for example, when seeing spectral analysis of distant stars,
> there is the rainbow which shows the colors representing a
> series of different elements, such as gases, which are shown
> in red, green, blue vertical bands of light, etc)...
>
Those are visible; if you use a prism or diffraction grating they can
actually be seen. The colors reflect the physical processes which
generate the light. Thus, if you add a small amount of copper to a
gas flame, the flame will turn green.
Sometimes color is also used to represent invisible relationships, of
course.
> if there is an electron in the atom, composing matter, the
> material world, and this has mass and energy, is electromagnetic,
> and some of its effects include giving off 'light' particles, photons,
> when a molecule or an atom's particles are knocked out of orbit,
> how does the photon, particle of light, relate to the electron,
> and these to the electromagnetic spectrum?
Physicists say that photons and electrons are of two different orders
of particles: bosons and fermions. Physicists say that the
distinguishing property of fermions and bosons is "spin"--fermions
have spins of 1/2, 3/2, and so on and bosons have spins of 0, 1, 2,
and so on--but I'm not sure what physicists mean when they talk about
"spin" at the subatomic level; it is not like the particles are
turning objects. At the same time, aggregate enough subatomic spin,
and one will observe macroscopic angular momentum--turning due to
magnetism. I'm not a physicist--I don't understand this. :-)
Anyhow, some of the simpler differences between electrons and photons are:
electron photon
has a rest mass no rest mass
has a charge no charge
participates in stable atoms carries electromagnetic energy
A photon can bump an electron up in orbital energy (by a
fixed--quantized--amount) and be absorbed. The new orbit isn't
stable, and eventually the electron falls back into its old orbit,
emitting another photon. There is no energy loss--there is no
friction at the sub-atomic level.
Electrons can also gain energy in other ways--for instance by
application of an external electric or magnetic field, or from
collisions with other sorts of particles. This is how electric lights
work.
Hope this is helpful,
Randolph